PHYSICAL AND CHEMICAL CHANGES :)!
So, todays class we had a lab! I found it very helpful for getting my eyes used to too seeing chemical and physical changes. With the different solutions being combined, we saw various results. From, bubbles being formed, to color change and to no changes at all.
Key Words:
Difference between physcial and chemical change.
Physical Change: Reversible; no new substance is formed.
Chemical Change: Irreversible; new substance is formed.
Chemistry Experiments: Watch the video for some interesting changes.
http://www.youtube.com/watch?v=ul4xRy8hcsQ&feature=related
**FOR LABS ALWAYS REMEMBER TO USE THE OUTLINE**
*REMINDER: CHAPTER 1&2 TEST OCTOBER 21, 2010**
I hope you guys enjoyed class today :)!
Wednesday, September 29, 2010
Tuesday, September 28, 2010
A Revision of Matter... yay!
Matter is anything that has mass and volume. It is not created or destroyed, it's only changed.
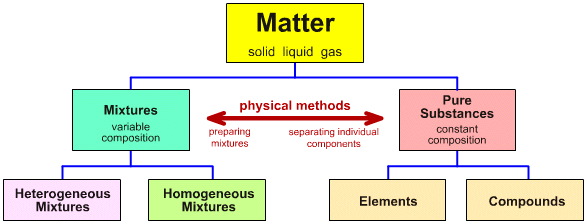
Matter can be divided up into two categories pure substances and mixtures.
First let's examine pure substances, they have only on kind of particle and therefore have one set of properties. They can be further categorized into elements and compounds.
Elements are in their simplest form, so they cannot decompose and are made of atoms. They can be a metals, non-metal or metalloid.
Compounds are made of elements which have been chemically combined, with ionic or covalent bonds. Their smallest particle is a molecule.
Mixtures have more than one kind of particle and therefore have more than one set of properties. Mixtures have been physically combined. They can be split up into two categories homogeneous and heterogeneous.
Homogeneous mixtures are uniform, they appear to only have one component. Ex: Salty water.
Heterogeneous mixtures are not uniform, you can see more than one component. Ex: Raisin Bread or oil and water. Heterogeneous mixtures can either be a suspension or mechanical mixture.
Change can be Physical or Chemical
Physical change: Does not produce a new substance and is mostly reversible. Ex: melting ice.
Chemical change: Creates a new substance, is irreversible. Signs of a chemical change are released gas, change in colour, precipitate formed and heat released (explosion). Ex: Burning paper.
THE VIDEO BELOW EXPLAINS THE THREE STATES OF MATTER
http://www.youtube.com/watch?v=s-KvoVzukHo
KP

Matter can be divided up into two categories pure substances and mixtures.
First let's examine pure substances, they have only on kind of particle and therefore have one set of properties. They can be further categorized into elements and compounds.
Elements are in their simplest form, so they cannot decompose and are made of atoms. They can be a metals, non-metal or metalloid.
Compounds are made of elements which have been chemically combined, with ionic or covalent bonds. Their smallest particle is a molecule.
Mixtures have more than one kind of particle and therefore have more than one set of properties. Mixtures have been physically combined. They can be split up into two categories homogeneous and heterogeneous.
Homogeneous mixtures are uniform, they appear to only have one component. Ex: Salty water.
Heterogeneous mixtures are not uniform, you can see more than one component. Ex: Raisin Bread or oil and water. Heterogeneous mixtures can either be a suspension or mechanical mixture.
Change can be Physical or Chemical
Physical change: Does not produce a new substance and is mostly reversible. Ex: melting ice.
Chemical change: Creates a new substance, is irreversible. Signs of a chemical change are released gas, change in colour, precipitate formed and heat released (explosion). Ex: Burning paper.
THE VIDEO BELOW EXPLAINS THE THREE STATES OF MATTER
http://www.youtube.com/watch?v=s-KvoVzukHo
KP
Friday, September 24, 2010
Even MORE Unit Conversion!
unit conversion quiz on Monday September 27th!
In the last post we learned about unit conversions! To recap unit conversions:
- convert 2 mg to grams
- start by writing out the equation:
- 2 mg x 10^3 mg/1 g
- to get 2 x 10^-3g or 0.002g
To continue with unit conversions, let us try a more complicated conversion:
- convert 10km/h to m/s
- we now have 2 units that need to be converted; km to m and h to min to s
- let's start by writing out the equation:
1h 1km 60min 60s
- we than cancel out units and multiply:
10km x 10^3m x 1hx 1min
1h 1km 60min 60s
- to get 2.77777778m/s or 2.8m/s
...a table of unit conversions!
have fun on the long weekend, and study hard!
-JY
Tuesday, September 21, 2010
Summary: Scientific Notation and Unitary Rates :)
Scientific Notation
-->Why do we use scientific notation?
-used to simplify and express really large or really small numbers using powers of 10
**3.26 X 107 (SCIENTIFIC NOTATION) = 32,600,000 (STANDARD FORM)**
Example: Express in scientific notation
a)5800 = 5.8 X 103
b)0.004= 4 X 10–3
Example: Express in standard form
a)6.3 X 104 = 63,000
b)7.9 X 10–6= 0.0000079
**MAKE YOUR LIFE EASIER BY USING A CALCULATOR FOR THE FOLLOWING TWO QUESTIONS :)**
*/*FOR PUNCHING IN EXPONENTS ON YOUR CALCULATOR USE 'E/Exp/X 10x'*/*
Evaluate(Final answer in scientific notation)
a)(4 X 104) + (9.8 X 106)
40000+9800000
=9840000
=9.84 X 106
b)(6.27 X 106) X (8.67 X 104)
=6270000 X 86700
=6356700
=6.3567 X 106
**WATCH THE VIDEO-IT HELPS!**
http://www.youtube.com/watch?v=lYWP1Fjmwhg
Unitary Rates
Example. If.....
1m=100cm
....What would
1m2 = ________cm2
Ans: 10,000cm2
Example: Convert
a) 9.2m3 to km3 *103m = 1km3
9.2m3 X 1km3/109m3 *109m3 = 1km3
=9.2 X 10–9km
**WATCH THE VIDEO**
http://www.youtube.com/watch?v=lDEaJBBVD1A
BLOG ENTRY #1-SEPT.21.2010
-->Why do we use scientific notation?
-used to simplify and express really large or really small numbers using powers of 10
**3.26 X 107 (SCIENTIFIC NOTATION) = 32,600,000 (STANDARD FORM)**
Example: Express in scientific notation
a)5800 = 5.8 X 103
b)0.004= 4 X 10–3
Example: Express in standard form
a)6.3 X 104 = 63,000
b)7.9 X 10–6= 0.0000079
**MAKE YOUR LIFE EASIER BY USING A CALCULATOR FOR THE FOLLOWING TWO QUESTIONS :)**
*/*FOR PUNCHING IN EXPONENTS ON YOUR CALCULATOR USE 'E/Exp/X 10x'*/*
Evaluate(Final answer in scientific notation)
a)(4 X 104) + (9.8 X 106)
40000+9800000
=9840000
=9.84 X 106
b)(6.27 X 106) X (8.67 X 104)
=6270000 X 86700
=6356700
=6.3567 X 106
**WATCH THE VIDEO-IT HELPS!**
http://www.youtube.com/watch?v=lYWP1Fjmwhg
Unitary Rates
Example. If.....
1m=100cm
....What would
1m2 = ________cm2
Ans: 10,000cm2
Example: Convert
a) 9.2m3 to km3 *103m = 1km3
9.2m3 X 1km3/109m3 *109m3 = 1km3
=9.2 X 10–9km
**WATCH THE VIDEO**
http://www.youtube.com/watch?v=lDEaJBBVD1A
BLOG ENTRY #1-SEPT.21.2010
Saturday, September 18, 2010
Subscribe to:
Comments (Atom)

