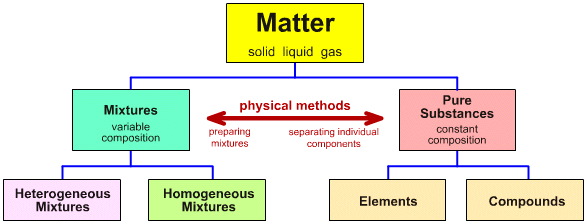
Matter can be divided up into two categories pure substances and mixtures.
First let's examine pure substances, they have only on kind of particle and therefore have one set of properties. They can be further categorized into elements and compounds.
Elements are in their simplest form, so they cannot decompose and are made of atoms. They can be a metals, non-metal or metalloid.
Compounds are made of elements which have been chemically combined, with ionic or covalent bonds. Their smallest particle is a molecule.
Mixtures have more than one kind of particle and therefore have more than one set of properties. Mixtures have been physically combined. They can be split up into two categories homogeneous and heterogeneous.
Homogeneous mixtures are uniform, they appear to only have one component. Ex: Salty water.
Heterogeneous mixtures are not uniform, you can see more than one component. Ex: Raisin Bread or oil and water. Heterogeneous mixtures can either be a suspension or mechanical mixture.
Change can be Physical or Chemical
Physical change: Does not produce a new substance and is mostly reversible. Ex: melting ice.
Chemical change: Creates a new substance, is irreversible. Signs of a chemical change are released gas, change in colour, precipitate formed and heat released (explosion). Ex: Burning paper.
THE VIDEO BELOW EXPLAINS THE THREE STATES OF MATTER
http://www.youtube.com/watch?v=s-KvoVzukHo
KP
No comments:
Post a Comment