OH! Why hello there,
I didn't see you come in.
Spoiler alert: this blog post is about alkenes and alkynes
but you probably knew that if you read the title.
but apparently it's cool if I pretend that we all didn't know
I'm sorry! I just want to fit in D:
Alkenes and Alkynes are carbons that can form double and triple bonds with carbon atoms respectively.
They have rules that are almost the same as alkanes (Position of bonds always has the lowest number and is put in front of the parent chain)
- one or more double bonds between carbon atoms
- lead to unsaturated hydrocarbon
- ending changed from -ane for alkanes to -ene for alkenes
- use the same general formula CnH2n
name this alkene
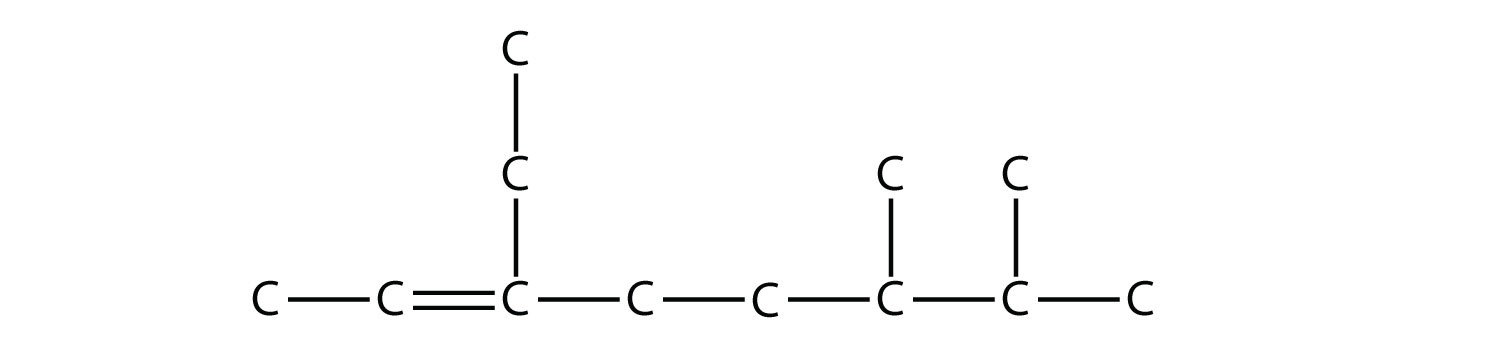 |
| 3-ethyl-6,7-dimethyl-2-octene |
- geometric isomers
- molecules that have the same structure but different geometry
- they must be distinguished by giving each a different name based on geometry
- compare groups attached to bonds
- If two adjacent carbons are bonded and have side chains on them 2 possible compounds are possible
- If the larger group is above (RR) or below (HH) the double bond is termed 'cis'
- If the larger groups are diagonal (RH), the double bond is termed 'trans'
Alkynes
- one or more triple bonds
- ending changes from -ane for alkanes, -ene for alkenes to -yne for alkynes
- general formula is CnH2n-2
- same naming rules but without the cis or trans
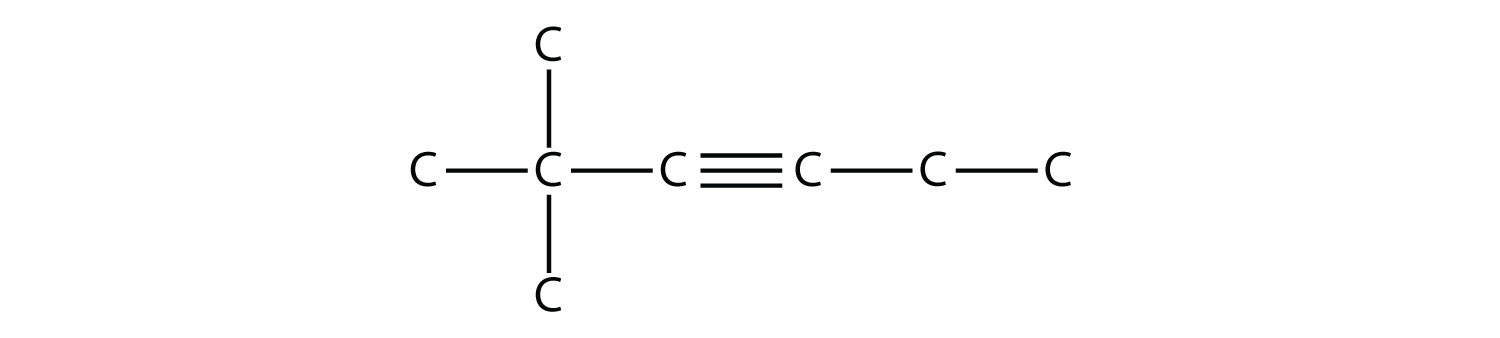
Practice: Draw 2,2-dimethyl-3-hexyne.
Name this alkyne.
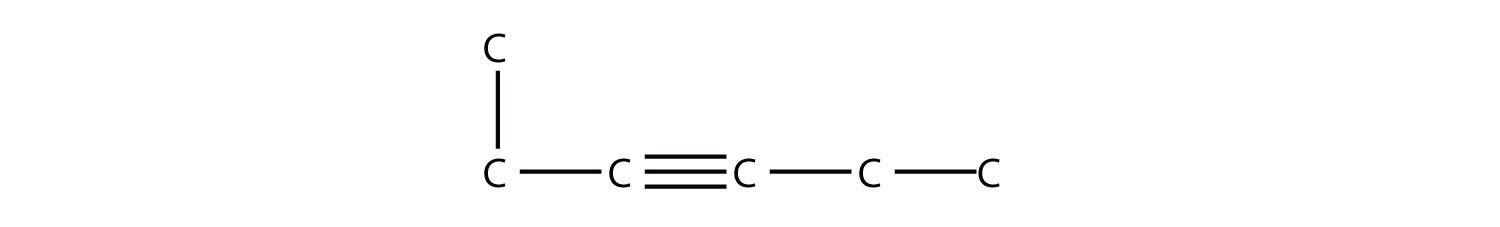 |
| 3-hexyne |
Ta DAH!
Now would you look at that? We finished a lesson even when I didn't hear you knock!
Here is a work sheet:
...
What? You're back??
and you STILL didn't knock?!