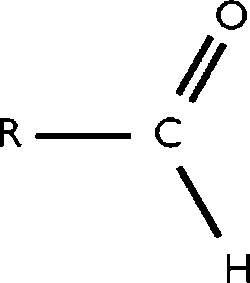
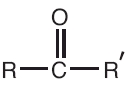
Throughout the decades many, many discoveries have been made by various individuals. Some ideas have grown from the help of newer generations of thinkers. ATOMIC THEORY is one of the concepts that, throughout the years has grown alot.
Greek philosophers suggested that matter was made up of atomos.
http://www.youtube.com/watch?v=ZnKqiojoFJU
- In 400 BC, Democritus was the first to propose that atoms were invisible particles.
-The came Aristotle, he proposed that matter was made up of earth, air, water, fire. He didnt not agree with Democritus's proposal. But his idea like Democritus wasnt proven because it was conceptual.
-Lavoisier stated the Law of Conservation of Mass and the Law of Definite Proportions. These laws suggested that in a compound of say, H2O, there will always be 11% Hydrogen and 89% Oxygen
-Joseph Proust experimentally proved Lavoisier's laws, and added that when a compound is broken down, products will exist in the same ratio as in the compound
-Then, in the early 1800s, John Dalton developed the basis of the modern Atomic Theory. He suggested that:
1. Elements were made of tiny indestructible spheres called atoms.
2. All atoms of an element were the same.
3. Atoms of a given element can be differentiated from another element by its relative atomic weights.
4. Atoms of one element will combine with atoms of other elements to create compounds.
-In the 1850's J.J Thompson created an experiment called the Raisin Bun Model. This model consisted of both positive and negative charges in a sphere like shape. With this he proposed that electrons existed.
-Ernest Rutherford explained why electrons spun around the nucleus, but he could not explain why the electron did not fall into the nucleus and destroy the atom.
-Thanks to Niels Bohr found a solution. Bohr was studying gaseous samples of atoms at the time, and came to the conclusion that electrons surrounding the nucleus were in specific energy levels. When the electron was excited, it would jump to a higher level. When an electron came back down, it would release energy in the form of light. Each of these jumps gives off light in different wavelengths; therefore creating different colours, as the colours ROYGBIV all have different wavelength
-an atom nowadays is considered the smallest particle of an element and cannot be broken down.
It contains 3 subatomic particles- the proton(+), the electron(-) and the neutron (0).
The protons and the neutrons occupy the nucleus and electrons exist in levels around the nucleus.
AND THATS A WRAP :)
enjoy this video....
http://www.youtube.com/watch?v=6p5nEhDv-cE&feature=related